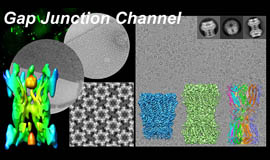
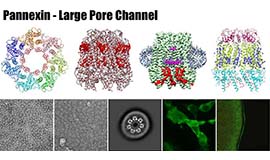
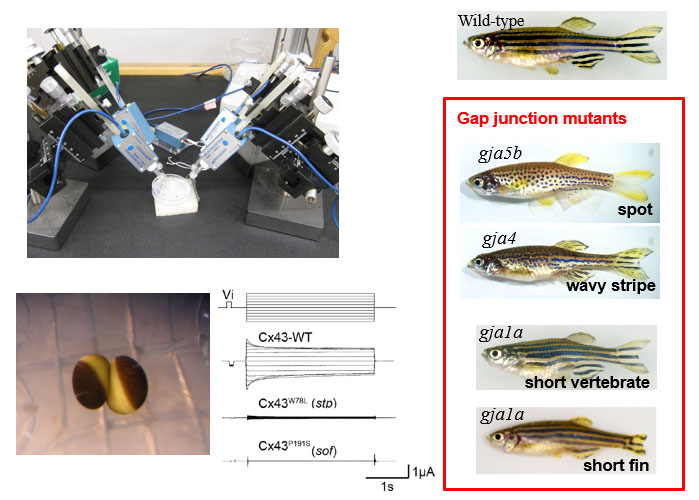
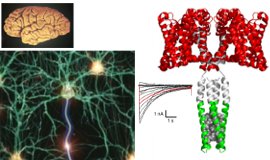
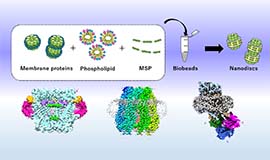
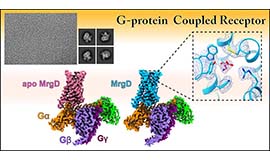
Intercellular communication is directly mediated by gap junction apparatus that forms conduits through adjacent two cell membranes in most multicellular organisms. Assembly of gap junction channels, termed gap junction plaques, mediates electrical and chemical coupling between cells, which is essential for various biological events such as development, inflammation, cell death, immune responses, and muscle contractions. We aim to understand the broad range of functional significance of gap junction channels in vivo. Cryo-EM is a powerful tool for high-resolution structure determination, and we will further integrate functional analysis and computational science to elucidate the gating mechanism of gap junction channels.